Aldehydes and Allergies
Aldehydes are so common and important in our lives that it’s good to know something about them. The flavor of vanilla, almond, cinnamon, and many others result from aldehydes. Aldehydes are a family of chemicals that are common in nature and foods, and important in industry. I knew that artificial vanilla and almond flavorings posed little danger to people with nut allergies. But the question came up in a discussion and I decided to find out if what I knew was, in fact, correct. (It is.) In the process of researching this, I fell down a rabbit hole of fascinating information, learned a number of interesting things related to chemistry and food chemistry, and collected it here in this article.
There is a family of chemicals called aldehydes that are extremely useful to plants and industry. It’s called a “family” because aldehydes have a common core molecular structure with an open bond to which various molecules can be attached that give various effects.
One thing aldehydes have in common is strong and distinctive odors. Another characteristic of aldehydes is that most of them are toxic in sufficient concentration. (Concentration is key here. At low concentrations, most of them are harmless.) I expect you are already familiar with or have heard of several of the aldehydes I’ll mention.
Formaldehyde
The one I’ll mention first is not closely related to foods but is one you’ve almost certainly heard of. Formaldehyde1 is a critically important chemical in many industries including plastics manufacturing, fibers, and adhesives. Some of the best and strongest waterproof glues are based on formaldehyde. Formaldehyde is a disinfectant and is used to preserve biological specimens. In biology class you may have seen specimens or body parts preserved in jars. Those were likely filled with formaldehyde as a preservative. Formaldehyde is toxic2 and carcinogenic in sufficient concentration. It’s produced in small amounts by most organisms, including humans.
You’ve all smelled it. It’s a key ingredient in “new car smell”, “new carpet smell”, and “newly constructed house smell”. Our noses are sensitive to aldehydes so very low concentrations are detectable.
Acetaldehyde
The next one is acetaldehyde.3 This one is produced by plants and occurs in bread, coffee, and ripe or overripe fruit, and is a component in the fragrance of wine and the smell of smoke. Acetaldehyde has a strong suffocating odor but at low-concentrations smells pleasant and fruity. Humans can detect acetaldehyde at 0.05 ppm. Diacetyl at a concentration of about 2 ppm with acetaldehyde at about 0.5 ppm are what give cottage cheese its flavor.
Acetaldehyde4 is formed from the oxidation of ethanol. It forms in the bloodstream after drinking alcohol and is partly responsible for hangovers.
⊂•⊃
Plants discovered the value of aldehydes a long time ago. When nature discovers a chemical that provides two unrelated benefits for the price of one, it’s a definite keeper. Aldehydes are such chemicals. Some of the most important flavors in foods result from aldehydes.
Vanilla
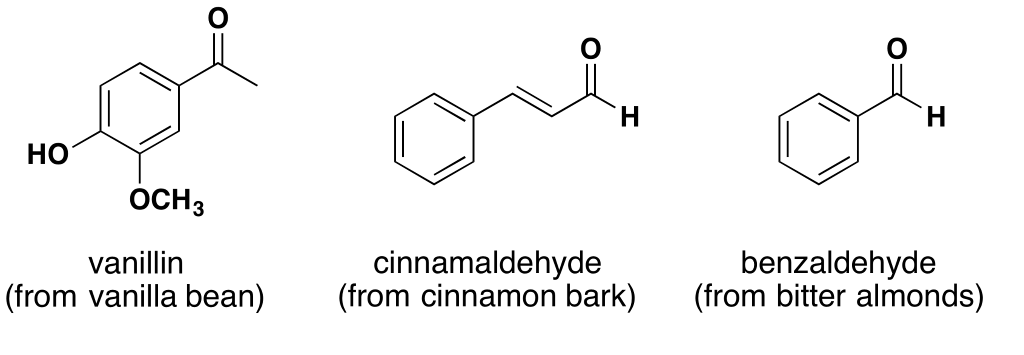
Vanillin5 is a member of the benzaldehyde family of aldehydes and is the chemical responsible for the flavor and fragrance of vanilla. It makes the seed pod of the vanilla orchid attractive to birds and animals and is an insecticide. Two functions for the price of one. Unlike most aldehydes, vanillin has low toxicity and is classed as merely an irritant.6
Vanilla has been in use in the Americas for at least 4,000 years. It was brought to Europe by Spanish explorers in the 1500s.
Cinnamaldehyde
The odor and flavor of cinnamon comes from an aldehyde that’s found in the bark of the cinnamon tree and various others where it serves as an insecticide. Cinnamaldehyde7 is a pale viscous liquid that functions as an anti-bacterial, anti-fungal, and is a potent repellent and killer of Aedes mosquitos. Like vanillin, cinnamaldehyde has low toxicity and is classed as merely an irritant.8
At high humidity and temperature, cinnamaldehyde decomposes to styrene. This is why cinnamon always contains a small amount of styrene.
Benzaldehyde9
This is the aldehyde that gives almonds their flavor.
It also occurs in certain fruits and the pits of peaches, apricots, apples, and cherries. It makes the fruit more attractive to animals and is an insecticide. It’s frequently used by bee keepers as a bee repellent. The odor causes bees to leave the hive while honey is collected after which they return to the hive.
Benzaldehyde is the simplest of all the aldehydes consisting of a benzene ring attached to the open bond of the aldehyde molecule. Benzaldehyde is classed as an irritant.10
Cuminaldehyde
Cumin, also known as cumino, comino, comyn, cymen, and many other names has been used as a spice / flavoring for at least 8,000 years. It’s common in Indian and Middle Eastern cooking. It was brought to the Americas by the Spaniards where it became an important ingredient in Latin American cooking. The the dominant flavor of chili and “taco meat” is usually cumin. The flavor of cumin comes partly from cymene and several terpenoids, but primarily from, you guessed it, cuminaldehyde.11.
⊂•⊃
Vanillin, cinnamaldehyde, and benzaldehyde are chemically classed as merely irritants. Technically all three are toxic in sufficient concentration, but so is water. To reach dangerous levels you’d have to consume absurdly large amounts. In the case of almond extract, you’d die of alcohol poisoning long before reaching dangerous levels of benzaldehyde.
The key here is concentration. Human noses are quite sensitive to aldehydes so for flavoring only a tiny amount is needed. Since most kitchens lack the ability to measure microgram quantities, these extracts are heavily diluted with alcohol so useful amounts can be measured with a teaspoon. At levels used for food flavoring there is some evidence these compounds provide anticarcinogenic effects.12
These aldehydes can all be synthesized in the laboratory or made inexpensively at industrial scale. There is no difference between vanillin made by an orchid and chemically produced vanillin. The same is true for benzaldehyde. If anything, the synthetic version gives a purer more consistent flavor note because it’s pure and not complicated by myriad other chemicals that vary from plant to plant.
Cilantro
The flavor of cilantro derives from several substances, some of which are aldehydes. Some people perceive the flavor of cilantro as a refreshing lemony-lime but some perceive it as tasting like soap or something rotten. It was found that 80 percent of identical twins had the same perception of cilantro but only 50 percent of fraternal twins did. This implied a genetic cause. Today the gene(s)13 have been identified. It’s believed that those who like cilantro are unable to detect one or more of the aldehydes in cilantro.
Furfural
Furfural is an aldehyde that results from the dehydration of sugars. It occurs in agricultural by-products like corncobs, oat bran, oat hulls, wheat bran, and sugarcane bagasse. It’s a common ingredient in processed foods and beverages. It commonly appears in many cooked or heated foods.
Furfural14 is classed as acutely toxic, an irritant, and health hazard.15
It’s dangerous to the skin. NIOSH permissible level is 5 ppm. 100 ppm is considered an immediate danger. Furfural is lethal to rats and dogs at concentrations of 200 to 1000 ppm. It’s flammable and explosive when mixed with air. It has a penetrating odor reminiscent of almonds. There is no data on human subjects.
Retinaldehyde
The importance of aldehydes cannot be overstated. Retinaldehyde16 is found in meats and is the chemical basis of our eye’s ability to sense light. Another name for it is Vitamin A aldehyde. Vertebrates acquire it directly from eating meat or can also synthesize it from carotenoids. In higher concentrations it’s classed as an irritant and a health hazard.17
Glycolaldehyde
This aldehyde is believed to play an important role in the formation of the chemical building blocks of life.18
Interestingly, radio astronomy has detected glycolaldehyde in interstellar space. It’s also been identified in Comet Lovejoy, along with ethanol. This aldehyde is classed as an irritant, highly reactive, and is a common metabolite produced by living things ranging from bacteria to humans.
Lily Aldehyde / Lysmeral / Lilial
Lily aldehyde19 has a strong floral odor reminiscent of lily of the valley. Thousands of tons are produced each year for use in perfumes and detergents.
It’s classed as a health hazard,20 found to be harmful to fertility, and is banned in the EU since March 2022.
Citral
If you’ve made it this far in this article, you can probably guess from the name that citral21 is the aldehyde primarily responsible for the fragrance of citrus fruits like lemon, lime, etc.
Citral is found to have a pheromonal effect on acari and insects. Chemically, it’s classified as an irritant.22
It’s a component in the oils of several plants, as follows.
Lemon myrtle (90–98%)
Litsea citrata (90%)
Litsea cubeba (70–85%)
Lemongrass (65–85%)
Lemon tea-tree (70–80%)
Ocimum gratissimum (66.5%)
Lindera citriodora (about 65%)
Calypranthes parriculata (about 62%)
Australian ginger (51-71%)
Petitgrain (36%)
Lemon verbena (30–35%)
Lemon ironbark (26%)
Lemon balm (11%)
Lime (6–9%)
Lemon (2–5%)
Citronellal
Citronellal is the aldehyde that gives citronella its lemony scent. Citronella23 is an insect repellent. Research has shown it to be highly effective at repelling mosquitos and is a strong antifungal.
Chemically, it’s classed as corrosive, an irritant, a health hazard, and environmental hazard.24
Allergies
Lastly, we come to allergies, specifically allergies to benzaldehyde or vanillin. It’s possible for a human to develop an allergy to almost anything, even metals like nickel. The good news is that it’s rare for someone to be allergic to these two aldehydes that are such important flavors.
The vast majority of allergies, whether it’s to pollen, animal dander, fish, shellfish, bee stings, whatever, are allergic reactions to proteins found in those things.
Natural almond extract is made by steaming almonds in a pressurized vat, then extracting the almond oil. This liquid includes not just benzaldehyde but many other chemicals including proteins. These proteins are usually what a person with a nut allergy reacts to. Genuine vanilla also contains many chemicals from the plant besides vanillin.
Artificial almond extract consists of a small amount of synthetic benzaldehyde diluted with ethanol. Neither has been anywhere near an almond tree and it contains no proteins or anything else. The same is true for artificial vanilla.
The concentration is chosen to match the flavoring power of genuine almond or genuine vanilla extract so the artificial is interchangeable with the genuine in recipes.
Fortunately, this means that those with nut allergies are almost sure to be safe with artificial almond or vanilla flavoring. Even so, if you have a nut allergy, especially a bad case, you should check with your doctor / allergist. They will be able to specifically test whether you are allergic to benzaldehyde (unlikely) or to the dozens of other compounds in nuts.
As a final note, a significant number of children and adults test positive for allergy to cilantro but only a few exhibit symptoms. For those who do, the symptoms can be severe.




Recent Comments