Lithium batteries are more dangerous and more delicate than I or my friends thought. It’s good to be aware of the dangers and treat them properly to minimize the danger.
For years now we’ve seen occasional reports of electric vehicles, cellphones, and notebook computers catching fire and sometimes burning spectacularly. It’s not common but it happens. One might say, “Oh, but that’s EV’s. That’s a special case.” It’s not. For the first several years, Tesla’s EV batteries were built out of 18650 lithium cells, 4,000 of them. These are the same cells found in power tool battery packs from Black & Decker, Porter Cable, DeWalt, and the rest. These are the same cells found in some notebook computers, flashlights, and many vaping devices.
There are several lithium battery chemistries. What I’m talking about here is the most common: NMC or Nickel Manganese Cobalt Oxide.

18650 refers to the physical size of the cell, 18 mm in diameter by 65 mm long. Other sizes exist but the 18650 is the most common shape.
An Incident Occurs
Like everyone else, I’ve been using lots of these batteries without issues. What caught my attention was an incident that happened a couple of weeks ago to a friend and co-worker. He got up in the morning to make coffee and noticed an odd smell. It smelled like overheated plastic, or failing electronics, or a pot left to burn dry on the stove. He walked back and forth in the house and determined the smell must be coming from his bedroom. Nothing was immediately apparent so he searched and found the cause. Under the bed was a plastic tote where he kept the batteries for his cordless garden tools. Since it’s been winter, those batteries had been there untouched for almost six months. Now, one of them was warm and plastic housing showed evidence of melted plastic and a hole where the plastic had boiled. He took the tote outdoors and called me. I went and took a look, took some photos, and noted the strong smell. Despite opening windows and ventilating the house, the smell lingered in the house for several days.
Later, he mentioned that the way the battery attaches to the tool is awkward and that he had dropped that battery on concrete from a height of about 1 meter. This cracked the plastic case. He thought nothing of it, glued the case together, and the battery continued to work normally. This news sent me digging on the Internet for information. I found quite a lot and learned some important things I didn’t know.
Dry Cells (Alkalines) Compared to Lithiums
Cylindrical lithium batteries like the 18650 are much more delicate than dry cell batteries. Dry cell batteries like alkalines are filled with a few simple bulky materials. Close manufacturing tolerances are not required and they are physically robust. Dry cell batteries can be dropped, dented, and partially crushed and won’t short-circuit. They usually continue to work. Even if a short somehow occurs, they fail gracefully. The maximum power they can deliver is limited and will not result in pyrotechnic jets of white hot flame or violent explosion.
Below is a table comparing old style dry cells introduced in 1898 , alkaline dry cells, and modern NMC lithiums. It shows the total energy content in MJ/kg (megajoules per kilogram), the total energy you can expect to get out of a cell in Wh/kg (watt-hours per kilogram), and the “specific power” the cell can deliver in W/kg (watts per kilogram). More on specific power below.
| Technology | MJ/kg | Wh/kg | W/kg |
| LeClanche Dry Cell (1898) | 0.13 | 36 | 10-27 |
| Alkaline Dry Cell (1949) | 0.13-0.68 | 85-190 | 50 |
| 18650 NMC (2008) | 0.74 | 205 | 3000-5100 |
From the above you can see that the differences aren’t that big except in one important way: watts per kilogram or specific power. Engineers use many terms like “specific power” that have agreed-upon meanings. In this case, specific power is the maximum amount of power a battery can produce for a short period of time. Short time meaning seconds or tens of seconds. Related terms would be instantaneous or pulse power (a fraction of a second) and continuous power (a long period of time or indefinitely).
As you can see, a lithium cell can deliver a hundred times as much specific power as an alkaline battery of the same weight. This jaw-dropping power to weight ratio is extreme, on the order of the engine in a top fuel dragster. Or, the engine in a Toyota Corolla producing 3,000 horsepower for a few seconds. It’s extreme. Over a period of a few seconds an 18650 can deliver enough power to heat itself smoking hot if it didn’t destroy itself in the process, which it would. This is the technology that enables the stunning performance of modern EVs.
At this point it should be obvious that short-circuiting an 18650 is a really bad idea. Just don’t. But what if an 18650 could somehow short-circuit itself? That would be really bad and is what we’ll talk about next.
Here’s the Problem
The construction of a lithium 18650 consists of paper-thin strips of film and foil rolled up like a jelly roll with a hundred paper-thin layers. Electronic engineers will recognize this type of construction from the way tubular paper, mylar, and electrolytic capacitors are made. This means that the positive and negative electrodes are very close together throughout the entire cell making it sensitive to dents, compression, bending, twisting, or any deformation from any direction. An unfortunate impact or dent can cause the cell to short-circuit, now or far in the future.
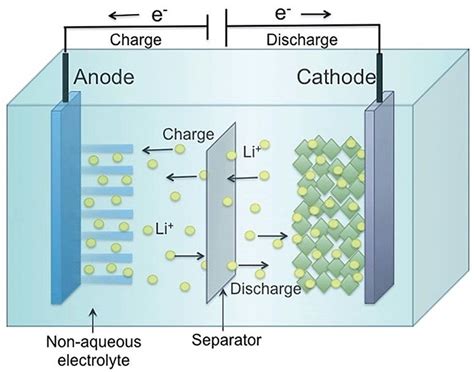
Making things worse is the formation of so-called “dendrites” in lithium batteries. For years, dendrites have been a mysterious problem that’s plagued lithium batteries. It refers to an effect where repeated charge/discharge of a lithium battery causes the growth of microscopic hairs or threads of lithium. These can short or partially short a cell, resulting in reduced battery life and sometimes catastrophic failure. Recent research has answered most of the questions about dendrites. An understanding of them will hopefully lead to better designs.
This recent research discovered that microscopic fissures in the insulating layer of the cell result in pathways for dendrite formation. Cracks, fissures, or perforations as tiny as 20 nanometers are a problem. (For reference, human hair ranges from 50,000 to 120,000 nanometers in diameter. So we’re talking flaws that are 3,000 to 6,000 times smaller than the diameter of a human hair.) Impacts, dents, or other deformations can result in such fissures which encourage the growth of dendrites.
In other words, if you drop or strike a lithium battery you may have started the clock on a ticking time-bomb. That’s what happened to my friend. He dropped the battery, damaging one or more of the cells, and a year later, after six months of storage, unused, under his bed, the battery short-circuited. Research into impact damage of 18650 cells shows that dents of 3mm and even smaller are a problem. Impacts, especially end-on impacts might show no visible damage, yet the damage is done internally and a time-bomb might start ticking.
Nearly all studies done over the past ten years examined the immediate effects of damage with the main focus on EVs. What happens in an automobile accident? Only recently have studies been done on the delayed effects of impacts and indentations.
One additional fact I discovered is that dendrite growth accelerates rapidly at temperatures above 65C or 149F. This temperature is easily reached and exceeded on the dashboard of a car in the summer sun. Keep your lithium batteries cool and out of direct sunlight.
Conclusion
I hope this information is useful. After learning these things I purchased a steel .50 caliber ammunition box where I now store my lithium battery packs. My box is made of thick steel and I hope this is enough to contain a catastrophic battery failure. It’s certainly better than the canvas kit bag I used to keep my batteries in.
These insights are disturbing. More and more flashlights have lithium batteries. I have three flashlights specifically designed to use an 18650 cell. Many vaping gadgets are powered by 18650s. Any handheld device might be dropped on a hard surface without the user thinking twice about it. And if they are aware of the problem, what then? Discard the battery and replace it or try your luck?
References:
https://www.sciencedirect.com/science/article/abs/pii/S0378775316314641
Recent Comments